New MGC OXYCAPT™
multilayer plastic vials
MGC’s new multilayer advanced material,
OXYCAPT™, integrates the best of plastic and
glass for plastic vials
What is OXYCAPT™? New plastic vial with both properties of glass and plastic
Traditional glass and plastic materials for syringes and vials are filled with problems. Glass suffers from a range of issues—such as high-breakability and poor PH stability—while plastic has an insufficient oxygen barrier and UV barrier. The US FDA and pharmaceutical companies have searched for solutions. Competitors have launched advanced material products, but their imperfect oxygen barriers and drug stability have been met with criticism.
This led to the creation of OXYCAPT™—MGC’s new, lightweight, multilayer material, with all the benefits of glass and plastic.
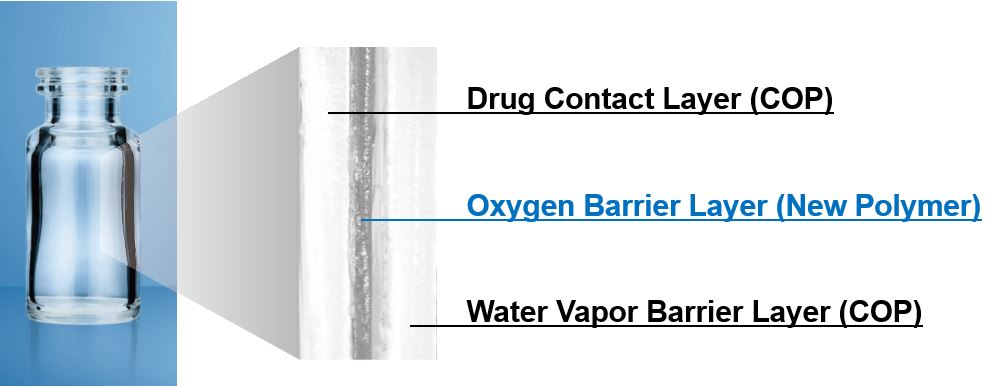
MGC’s OXYCAPT™ unites the best qualities of glass and plastic in a three-tiered, multilayer, advanced material that features an excellent water vapor barrier & drug contact layer made from COP (Cyclo Olefin Polymer) and an oxygen barrier layer with an oxygen barrier polymer. With low extractables, low protein absorption, and low breakability, all components come together to produce the best high oxygen barrier material on the market.
*The white paper including heat cycle test at -80 to +40, CCI test at -80 & -180℃, CO2 barrier after dry ice storage, etc. has been posted on the website of Drug Development & Delivery.
https://drug-dev.com/whitepapers/whitepaper-oxycapt-multilayer-plastic-vial-an-optimal-primary-container-for-drug-products-transported-with-dry-ice/
Multilayer Configuration
Why it’s betterA breakthrough in longer-lasting, safer medications
OXYCAPT™ provides high oxygen & CO2 barrier —along with the low inorganic extractables, low breakability, and lightweight properties of plastic. Inorganic low molecular extractables are below that of Type I glass. Excellent UV absorption means drugs last longer, with improved stability and efficacy throughout their lifespan.
Also for biologics and biosimilars, OXYCAPT™’s low extractables, high-breakage resistance, and high oxygen barrier—plus an improved UV barrier that absorbs UV and preserves drugs longer—make it the best, advanced material. Pharmaceutical companies with gene/cell therapy products also see benefits.
| Glass | COP (Cyclo Olefin Polymer) |
OXYCAPT™-P | |
|---|---|---|---|
| Oxygen & CO2 Barrier | Excellent | Not Good | Good |
| Water Vapor Barrier | Excellent | Good | Good |
| Resistance to Breakage (Normal Temp.) |
Bad | Good | Good |
| Resistance to Breakage (Deep Cold Temp.) |
Bad | Not Good | Good |
| Conteiner Closure Integrity (Deep Cold Temp.) | Bad | Not Good | Good |
| Inorganic Extractables | Not Good | Excellent | Excellent |
| Organic Extractables | Excellent | Excellent | Excellent |
| Protein Adsorption | Not Good | Good | Good |
| pH Stability | Not Good | Good | Good |
| UV Barrier | Bad | Bad | Good |
| Weight | Bad | Excellent | Excellent |
| Disposability | Bad | Good | Good |
| Heat Resistance | Excellent | Good | Not Good |
* Excellent − Good − Not Good − Bad
OXYCAPT™ in useThe best choice for biologics and cell/gene therapy products
MGC’s OXYCAPT™ plastic vials are the right solution for parenteral pharmaceutical liquid medication storage. OXYCAPT™’s multilayer construction preserves drug stability and shelf life in plastic vials, with significantly reduced oxidation, compared to COP. Long-term trials find OXYCAPT™ eliminates the problem of delamination (visible small flakes and particles from glass deterioration over time) in glass, while maintaining its oxygen barrier. OXYCAPT™ also resolves poor stability and low visibility issues found in plastics. In addition, OXYCAPT™'s CO2 barrier and resistance to deep-cold storage have been appreciated by pharmaceutical companies because next generation drugs stored at cryogenic temperature and transported with dry ices have become popular recently.

The OXYCAPT™ AdvantageOutperforming plastic with superior material
Oxygen & CO2 Barrier
OXYCAPT™-P Vial has high oxygen & CO2 barrier property. The oxygen & CO2 barrier is about 20 times better than COP. The result ensures consumers, pharmaceutical companies, biopharmaceutical companies, and entrepreneurial ventures looking to deliver biosimilars, biomedicines of advanced biologics, and cell/gene therapy products, receive effective medication after longer periods of storage time.
10ml Vial
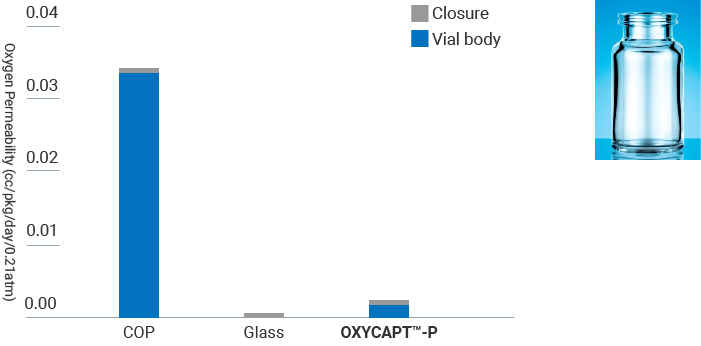
- Measurement Device : MOCON OX-TRAN® 2/61
- Condition : 23℃ / In 100%RH, Out 50%RH
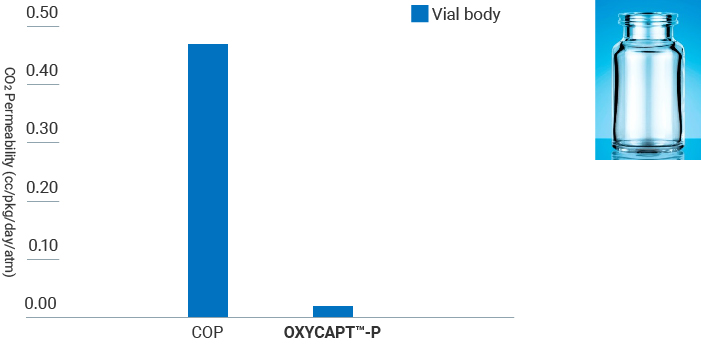
- Measurement Device : Lighthouse Instruments FMS-CO2*
- Storage Condition:23℃ / 0%RH
- Measurement Condition : 23℃ / 50%RH
*CO2 partial pressure measured by FMS-CO2 was converted into CO2 permeability.
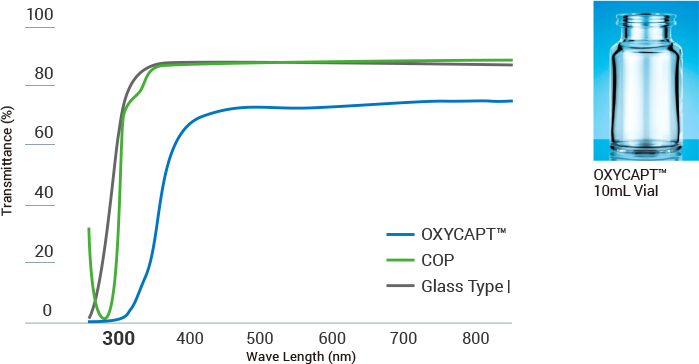
UV Barrier
Pharmaceutical companies heavily focus on improving UV barriers of packaging container materials because UV deteriorates drug contents and stability. Through UV absorption, OXYCAPT™ protects drugs better than COP and Glass Type I by cutting off UV light below 300 nm.
Inorganic Extractables
Increased scrutiny by regulators has led the medical, pharmaceutical and biopharmaceutical industry to focus on inorganic extractable assessments to ensure patient safety and drug interaction. OXYCAPT™ outperforms all plastic and glass advanced materials—including Type 1 Glass, COP, PP, and PE—currently on the market.
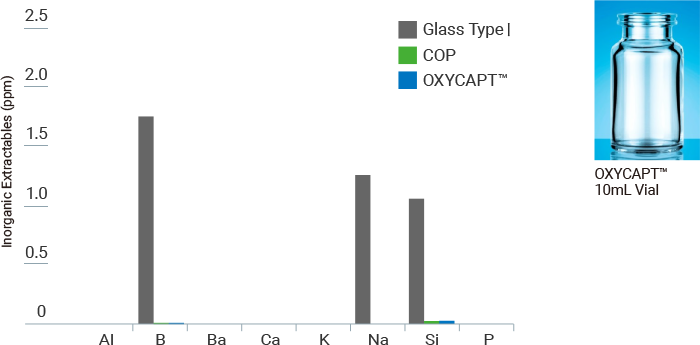
<Test method>
- - Filled with 10mL Water
- - Stored for 1 Month(25℃/60%RH)
- - Analysis; ICP
Product Portfolio
| ISO Vial |
Height (㎜) |
OD of Body (㎜) |
OD of Crown (㎜) |
ID of Crown (㎜) |
Packaging Option |
|---|---|---|---|---|---|
| 2R (2mL) | 35 | 16 | 13 | 7 | Tray or Nest/Tub |
| 6R (6mL) | 40 | 22 | 20 | 12.6 | Tray or Nest/Tub |
| 10R (10mL) | 45 | 24 | 20 | 12.6 | Tray or Nest/Tub |
| 20R (20mL) | 55 | 30 | 20 | 12.6 | Tray or Nest/Tub |
*The product is customizable. Please contact “Contact Form”.

Inquiries Concerning Products
Research & Development Division
Health Technology & Solution Department
TEL:+81-3-3283-4915